Ultrasound Guided Percutaneous Peripheral Nerve Stimulation: A Nonpharmacological Alternative for the Treatment of Postoperative Pain
Project Summary
This project encompasses two multi-center, randomized, double-masked, placebo-controlled, parallel-arm clinical trials. A two-year feasibility study estimated the treatment effect of percutaneous peripheral nerve stimulation (PNS) on pain and opioid consumption following painful ambulatory surgery. The results of this study determines the sample size of the subsequent pragmatic clinical trial, as well as documenting the feasibility and optimizing the proposed protocol for the subsequent four-year pragmatic clinical trial. In this trial, we determine the effect of percutaneous PNS on postoperative analgesia and opioid requirements, as well as physical and emotional functioning, the development of chronic pain, and ongoing quality of life.
Principal Investigator

Brian Ilfeld, MD, MS
Impact and Contribution to PMC and Society
This investigation has a strong potential to dramatically reduce or obviate postoperative opioid requirements and their resultant negative effects on both individuals and society; while concurrently improving analgesia, increasing the ability to function in daily life, decreasing the risk of transition from acute to chronic pain, and improving quality of life. If successful, percutaneous PNS could revolutionize postoperative analgesia as it has been practiced for the past century.
Details
Institutions: University of California, San Diego
Sponsor: Department of Defense, Congressionally Directed Medical Research Programs (CDMRP)
For an overview, visit clinicaltrials.gov NCT03481725
For results go to publications and search for Ultrasound Guided PNS trial
Project Narrative
There are tens-of-millions of ambulatory surgical procedures performed in the U.S. annually, with many of these in active-duty military, veterans, and their family members. Postoperative pain is usually treated with opioids that have undesirable and sometimes dangerous side effects (e.g., vomiting and respiratory depression)—and yet over 80% of patients still experience inadequate pain relief. Moreover, opioids have a significant potential for diversion, abuse, dependence, and overdose, with wounded service members and veterans disproportionately affected. Within the U.S. Armed Services, the most-commonly abused class of prescription drugs is opioids, with the incidence of misuse increasing from 2% to 11% between 2002 and 2008, a trend that has not abated. The Army has recently instituted a policy to limit opioid prescription use due to the rising rates of abuse to what most now describe as an epidemic.
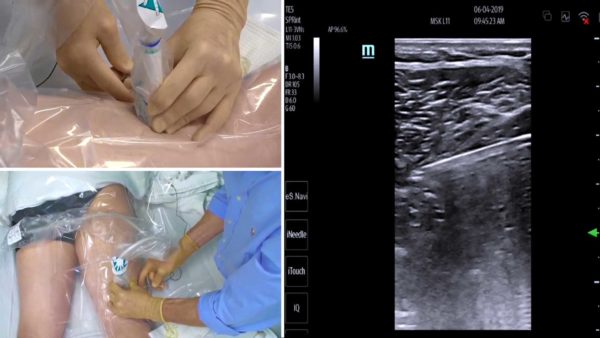
A novel, non-pharmacologic analgesic technique—percutaneous peripheral nerve stimulation (PNS)— holds extraordinary potential to greatly reduce or obviate opioid requirements and concurrently improve analgesia following painful surgery. This technique involves inserting an insulated electric lead adjacent to a target nerve through a needle prior to surgery using ultrasound guidance. Following surgery, a tiny electric current is delivered to the nerve resulting in potent pain control without any cognitive or adverse systemic side effects whatsoever. The electrical pulse generator (stimulator) is so small it is simply affixed to the patient’s skin. The leads are already cleared by the U.S. Food and Drug Administration to treat acute (postoperative) pain for up to 30 days; and, since percutaneous PNS may be provided on an outpatient basis, the technique holds the promise of providing potent analgesia outlasting the pain of surgery—in other words, the possibility of a painless, opioid-free recovery following surgery.
More from Dr. Brian Ilfeld
Video: Ultrasound-Guided Peripheral Nerve Stimulation for Postoperative Pain Management Brian Ilfeld, MD, MS, talked with the Pain Management Collaboratory Coordinating Center about his pragmatic clinical...Read More >>
Brian Ilfeld, MD, is studying the use of ultrasound-guided peripheral nerve stimulation to treat postoperative pain after ambulatory surgery, specifically after shoulder and ankle and...Read More >>